আধুনিক রসায়ন এর প্রতিষ্ঠাতা জন ডাল্টনঃ
The fundamental idea of modern chemistry is that matter is made up of atoms of various sorts, which can be combined and rearranged to produce different, and often novel, materials. The person responsible for "this master-concept of our age" (Greenaway, p. 227) was John Dalton. He applied Newton's idea of small, indivisible atoms to the study of gases in the atmosphere and used it to advance a quantitative explanation of chemical composition. If French chemist Antoine Lavoisier started the chemical revolution, then it was Dalton who put it on a firm foundation. His contemporary, the Swedish chemist Jöns J. Berzelius, said: "If one takes away from Dalton everything but the atomic idea, that will make his name immortal."
John Dalton was born on or about September 6, 1766, to Quaker parents, in Eaglesfield, a remote village in the north of England. He was largely self-educated, and learned most of his mathematics and science by teaching others. He studied mathematics in a local school until the age of 11, started his own school at the age of 12, and at 15 joined his brother Jonathan in teaching at, and later running, a Quaker school in Kendal. The Quakers were a small dissenting (from the established Church of England) sect, and Dalton was thus a nonconformist, like the scientists Joseph Priestley and Michael Faraday. Dalton was taught and influenced by fellow Quakers Elihu Robinson, a wealthy instrument maker, and John Gough, a blind polymath. In Kendal Gough taught the young Dalton Latin, Greek, French, mathematics, and science, and in return Dalton read to him from books and newspapers. Gough encouraged Dalton to study natural phenomena and to keep a meteorological journal, which Dalton began on March 24, 1787. Dalton maintained this journal methodically for the rest of his life, making his last meteorological observations on his deathbed. He made over 200,000 measurements over a period of fifty-seven years, and a neighbor in Manchester is supposed to have said that she was able to set her clock by Dalton's daily appearance to take the temperature. Dalton's meteorological observations launched his scientific career and provided the material for his first book, Meteorological Observations and Essays (1793).

British chemist and physicist John Dalton, who drew up the first list
of atomic weights.
Dalton stayed in Manchester for the rest of his life, and it was there that he did most of his important work, the results of which were published in the Memoirs of the Manchester Literary and Philosophical Society (MLPS). His first scientific paper, published by the MLPS in 1798, described his red-green color blindness. Dalton is said to have purchased for his mother a pair of what he thought were dull-colored stockings—Quakers did not wear bright colors—which she could not wear because they were scarlet. This misadventure motivated Dalton to investigate his color recognition deficiency. He was the first to describe red-green color blindness, sometimes known as Daltonism.
Dalton's study of the atmosphere, prompted by his weather measurements, led him in 1803 to his law of partial pressures (in a mixture of gases, each gas acts as an independent entity), and subsequently to the study of the combining of elements. He compared marsh gas (methane, CH 4 ) with olefiant gas (ethane, C 2 H 4 ), and found that ethane contained exactly double the mass of carbon to the same mass of hydrogen. It is this relationship between the two gases that guided him to his law of multiple proportions. He imagined a chemical atomic model, whereby one atom of an element could combine only with one, two, or three atoms (and so on) of a second element, the combinations forming distinct compounds. He visualized atoms as small hard balls and constructed small wooden models to illustrate how they combined. He invented symbols that enabled him (and others) to notate chemical formulas ✷. Dalton drew up the first list of atomic weights. Dalton's ideas about atoms and their combinations were first aired in 1803 at meetings of the MLPS, mentioned in Thomas Thomson's System of Chemistry (1807), and finally published by Dalton in his most important book, New System of Chemical Philosophy (1808).
অণু ধারণার প্রবর্তক এ্যামেদেও অ্যাভোগাড্রোঃ
The Avogadro Project
The kilogram is the only remaining fundamental unit within the International System of Units (or SI, from Système International d'Unités in French) which is defined in terms of an artifact. This mass standard comes in the form of a Pt-Ir cylinder kept in the International Bureau of Weights and Measures (or BIPM, from Bureau International des Poids et Mesures in French) situated in Paris. The proposition of the Avogadro Project is to redefine the kilogram in terms of the Avogadro constant.
 By
definition, an Avogadro number of Carbon-12 atoms weigh exactly
12 grams. As such, the kilogram could bedefined as the mass of
1000/12 * Avogadro's number of Carbon-12 atoms. The Avogadro constant
itself is obtained from the ratio of the molar mass to the mass
of an atom. For a crystalline structure such as silicon, the atomic
volume is obtained from the lattice parameter and the number of
atoms per unit cell. The atomic mass is then the product of the
volume and density.
By
definition, an Avogadro number of Carbon-12 atoms weigh exactly
12 grams. As such, the kilogram could bedefined as the mass of
1000/12 * Avogadro's number of Carbon-12 atoms. The Avogadro constant
itself is obtained from the ratio of the molar mass to the mass
of an atom. For a crystalline structure such as silicon, the atomic
volume is obtained from the lattice parameter and the number of
atoms per unit cell. The atomic mass is then the product of the
volume and density.The Avogadro Project involves an international collaboration between laboratories in Germany, Italy, Belgium, Japan, Australia and USA. Currently the Avogadro constant is known to an uncertainty of approximately 0.1 ppm. It is hoped that the uncertainty will be reduced to 0.01 ppm after a further five years.
In determining the Avogadro constant, the preferred method has been to use one of the high-precision spheres fabricated here at the ACPO. These come in the form of a highly polished 1 kg single crystal silicon sphere, fabricated with a roundness in range of 60 nm. Silicon is used because of its well known crystal structure, stability and its relative ease of use. The volume is determined from the measurement of the silicon sphere's diameter and roundness. Accurate measurement of the mass then allows the density to be derived.
 The
nominal diameter of a 1 kg Si sphere is 93.6 mm. In order to obtain
an accuracy of 0.01 ppm in volume, the diameter must be known
to a range of 0.6 nm. In other words, within one atom spacing.
Such high accuracy requires specialised equipment and one such
procedure is by optical interferometry using a precision etalon
through a stabilised laser light. The measurements are sensitive
to many parameters, particularly to those of temperature and pressure.
An instability within the range of 2 mK would be sufficient to
cause the silicon to expand by more than the allowable uncertainty.
The refractive index of air (and hence the wavelength of the light)
is sensitive to the surrounding air pressure. It is therefore
necessary to carry out the measurements in a controlled environment.
The
nominal diameter of a 1 kg Si sphere is 93.6 mm. In order to obtain
an accuracy of 0.01 ppm in volume, the diameter must be known
to a range of 0.6 nm. In other words, within one atom spacing.
Such high accuracy requires specialised equipment and one such
procedure is by optical interferometry using a precision etalon
through a stabilised laser light. The measurements are sensitive
to many parameters, particularly to those of temperature and pressure.
An instability within the range of 2 mK would be sufficient to
cause the silicon to expand by more than the allowable uncertainty.
The refractive index of air (and hence the wavelength of the light)
is sensitive to the surrounding air pressure. It is therefore
necessary to carry out the measurements in a controlled environment.High purity silicon boules have been produced especially for this project by Wacker in Germany. The silicon is produced by the float zone process and a very small quantity of nitrogen is introduced to minimise defects, but at a concentration sufficiently low as to not affect the molar mass. The determination of the molar mass is conducted though mass spectroscopy.
Corrections must be applied for surface impurities such as oxides and absorbed water. Typically, silicon has an oxide layer 3 to 4 nm thick, which is a mixture of SiO and SiO2. It is also possible for the surface to absorb some monolayers of water. Since much of the absorbed water is removed in a vacuum, a number of the key measurements are made in a vacuum environment. A further correction must then be applied for the difference in bulk modulus between the air and vacuum.
Before a permanent and absolute definition of the kilogram is introduced, the relative stability of the silicon sphere and the existing Pt/Ir aftifact will have to be monitored. The kilogram can then be defined in terms of a specific number of Carbon-12 atoms.
আধুনিক রসায়নের উন্নতিকল্পে রাদারফোর্ডের ভূমিকা অনস্বীকার্যঃ
Rutherford's Model of the Atom
Although some ancient Greeks (such as Democritus) postulated the existence of atoms (units of matter which could not be subdivided), concrete evidence for their existence did not develop until the 19th century. The first direct evidence came from observations of the Brownian motion.
Other evidence came from chemistry, such as the experiments of Faraday on electrolysis (1833). Faraday's law states that when a current is passed through a solution or through a molten electrolyte, the mass of a particular element deposited at the cathode or anode is proportional to the electrical charge which has flowed around the circuit (current times time) and to the atomic weight of that element, but inversely proportional to the valence of the element. Although its interpretation was not clear at the time, Faraday's law reflects the fact that electricity passes through an electrolyte in the form of ions whose mass is proportional to the atomic weight and whose charge is equal to the valence, which represents the number of electrons which have been removed from the neutral atom.
The properties of electrons were investigated by J.J.Thomson at the Cavendish Laboratory in 1897 (Figs. 3.2 - 3.4). An electrical discharge in a low-pressure gas was known to produce cathode rays, which could cause an object in their path to emit light (fluoresce), but it was not known if these rays consisted of waves or material particles. Thomson passed a beam of cathode rays through a uniform electric field E (between two parallel plates) and a magnetic field B (produced by an electromagnet) acting over the same region of space (see Fig. 3.3).
The two fields were perpendicular to the beam and to each other, such that the magnetic and electrical forces were both perpendicular to the beam but opposite in direction. By adjusting the strength of one of the fields, so as to produce zero net deflection as observed on a fluorescent screen, he achieved the condition:
e E + B e Vx = 0
and so was able to measure the speed Vx of the particles in the beam (travelling in the x-direction). With the magnetic field turned off (Fig. 3.5), the electric field produced a force (and therefore an acceleration a) on each particle over a distance L, deflecting the beam through an angle q which could be measured. The magnitude Vy of the velocity acquired in a direction parallel to the field (i.e. perpendicular to the beam) can be calculated from
Vy = a t = (eE/m) (L/Vx) , so that: tan (theta)
= Vy / v = (L E / Vx^2) (e/m)
By measuring (theta)
, L and E (=V/d where V is the voltage between the plates, separation d) the charge-to-mass ratio e/m of the particles could be found.The value of e/m was found to be independent of the electrodes and the gas in the discharge tube, suggesting that the cathode rays consisted of particles which were a constituent of all matter. The value of e/m was about 2000 times larger than the charge/mass ratio measured (by electrolysis of water) for hydrogen ions, indicating a particle much smaller in mass than the smallest atom.
The electronic charge e was first determined by Robert Millikan (in 1909) by observing the motion of individual drops of oil in an vapour, ionized with a radioactive source; see Fig. 3.7. A drop acquires a terminal velocity as a result of a balance of the viscous(air-resistance), gravitational and (with an electric field applied) electrostatic forces. Applying a formula for the viscous force on a spherical drop and measuring the velocity with an without applied field, the charge on the drop can be calculated.
Rutherford Scattering
Although atoms were originally thought of as being indivisible, evidence began to accumulate (towards the end of the 19th century) that the atom contains component parts: electrons (which can be emitted as cathode rays) and a balancing positive charge (to give overall neutrality and prevent the atom exploding from the electrostatic repulsion of all the negatively charged electrons).
In 1898, J.J. Thomson proposed that the electrons are embedded (like plums in a pudding) in a sphere of uniformly distributed positive charge. Other physicists came up with other ideas, for example the positive charge might be concentrated in a central nucleus with the electrons orbiting around it, analagous to the solar system, except that the attractive forces would be electrostatic rather than gravitational.
Experiments conducted between 1909 and 1924, supervised by Ernest Rutherford but actually carried out by two of his students (Hans Geiger and Ernst Marsden), provided the evidence necessary to choose between these models. The experiments arose out of investigations of radioactive materials, discovered by Becquerel in 1896 when he accidentally left wrapped photographic plates in a drawer together with some uranium-salt crystals (he shared a 1903 Nobel prize with Pierre and Marie Curie).
The apparatus was quite simple: a radioactive source (such as radon gas) emitted alpha particles, which have a charge of +2e and a mass about four times that of the hydrogen atom (they are actually helium-atom nuclei). A parallel beam of these particles (collimated by a lead tube) was directed towards a thin foil of a metal such as gold, mounted in a vacuum chamber. Some of the alpha particles passed through the foil without measurable deviation but some were scattered through appreciable angles (j in Fig.3.9). The angular distribution of the scattered a -particles was measured by mounting a fluorescent screen (a slab of glass coated with fine zinc suphide particles) at the entrance of a low-power telescope, which could be rotated about the scattering point; see Fig. 3.9. With a dark-adapted eye, single a -particles hitting the screen could be detected as a small flash of light seen in the telescope. Most of the alpha particles were deflected through small angles (of the order of 1 degree) but a small fraction were scattered through larger angles, including some backscattered through angles exceeding 90 degrees = p /2 radians; see Fig. 3.11.
Rutherford realized that the existence of large-angle scattering ruled out the Thomson (plum-pudding) model; the relatively heavy a -particles would not be turned around by much lighter electrons or by the combined mass of a gold atom if this mass were distributed over the whole atomic volume. On the other hand, if the positive charge and most of the mass of a gold atom were concentrated in a central nucleus, its electrostatic repulsion would repel incoming a -particles and deflect some of them through angles as large as 180 degrees (see Fig. 3.10) without absorbing much of the a -particle's energy (i.e. the "collision" will be elastic). This scattering from the electrostatic field of the nucleus is now known as Rutherford scattering. Note that if the nucleus is small compared to the whole atom (it typically occupies less than 1 part in 10^15 of the volume!), the probability of high-angle a -scattering will be very small, in agreement with the measured angular distribution (note the logarithmic vertical scale in Fig. 3.11).
Based on his nuclear model of the atom, Rutherford was able to calculate an expression for the angular distribution of the a -particle scattering. He needed to know the magnitide F of the force on an a -particle when it is a distance r from the centre of a nucleus; this is given by Coulomb's law:
F = k (+2e)(Ze)/r^2
where k is the Coulomb constant
and (Ze) is the nuclear charge, Z being the atomic number of the atoms in the foil. Applying Newton's second law
(and conservation of momentum and energy) to the two-particle interaction led to the following expression for the number
n of alpha particles detected at a scattering angle (phi) :
n = C ( N Z^2 / K^2 ) / [sin (phi)/2]^4
where K is the kinetic energy of the alpha
-particles and C is a parameter which depends on the strength of the alpha
-particle source and the geometry of the particle detector; N is the number of nuclei per unit area of the foil, equal to (rho) t / (A u)
where t and (rho) are the foil thickness and density, while A represents atomic weight and u is the atomic-mass unit.
Careful experiments by Geiger and Marsden, published in 1913 (Philosophic Magazine 25, p.605), confirmed the t, K and
phi dependence implied by this formula. At that time, atomic numbers for different elements were not known; however, Rutherford was able to fit the results obtained from different foils to his formula by trying different values of Z ; see Fig. 3.11. Thereby, he was able to measure Z , while the relatively good degree of fit provided additional confirmation of his theory.
Furthermore, Rutherford realized that the remarkable success of his formula provided information about the size of the nucleus. He argued that if an alpha actually reached the nucleus, the latter would be "deformed" - in other words the force law would depart from the Coulomb's law expression and the angular dependence of scattering would change. A particle which gets closest to a nucleus will be one which directly approaches its centre and is deflected through 180 degrees (as in Fig. 3.10). At the moment of its closest approach, this particle will be momentarily stationary, having exchanged all of its kinetic energy K into electrostatic energy of repulsion, so that:
K = k (Ze) (2e) / r
Smaller values of r will be possible by choosing foils of lower atomic number and alpha particles of higher kinetic energy. The experimenters therefore worked with aluminum foils and other radioactive sources which provided more penetrating radiation, looking for evidence for departures from Rutherford's formula in the measured angular distributions.
In 1919, they were able to detect a departure from the formula for 7.7MeV
alpha-particles (K = 7.7 x 10^6 / 1.6 x 10^-19 Joule) scattered from a foil of aluminum (Z=13), allowing the radius of the Al nucleus to be estimated as: r = 2 k Z e^2 / K = 4.9 x 10^-15 m. Since the radius of an atom is of the order of 10^-10 m, the atom is seen to be mainly empty space.Despite the success of the nuclear atomic model, it raised several questions. For example, the atomic number (a measure of the nuclear charge) of an element turned out to be more than a factor two lower than its atomic weight (a measure of the nuclear mass mass). Rutherford speculated that the nucleus might contain electrons, which would neutralize some of its positive charge without adding appreciable mass. This seemed plausible at the time, because certain types of radioactive materials emit high-energy electrons (beta- rather than alpha-decay) which might come from the nucleus.
Rutherford thought that the presence of electrons might explain another problem with the nuclear model: why does the nucleus not fly apart because of electrostatic repulsion of the positive particles? Perhaps the electrons act as a kind of glue which holds the nucleus together ? Only later did it become clear that, at the close separations involved in the nucleus, its component particles exhibit strong nuclear forces which completely overwhelm the electrostatic repulsion and which represent an entirely different kind of interaction.
Probably the most serious problem with the planetary model is that an orbiting electron has a centripetal acceleration and (according to Maxwell's theory of electromagnetism) ought to lose energy by emitting electromagnetic radiation at a frequency equal to that of the orbital motion (the reciprocal of the orbital period). This radiated energy would be at the expense of the electrostatic potential energy of the electron, which would become more negative - implying that the electron approaches closer to the nucleus and experiences an increased electrostatic force. This increased force implies an increased centripetal acceleration and a higher angular velocity of the orbiting electron; the frequency of the emitted radiation would increase and the electron would spiral into the nucleus, as indicated in Fig. 3.20. Calculations showed that this process should happen in a small fraction of a second; in other words, the atom should not be stable ! The problem was not solved by Rutherford; it took the genius of Niels Bohr to propose a solution.
3D PHOTOS OF ORBITALS:
Atomic Orbitals
Electron orbitals are the probability distribution of an electron in a atom or molecule.10 April 2001: A minor update to Orbital Viewer has been posted.
| A brief description of atomic orbitals (below). | |
A program for drawing orbitals. This has many features, and comes in
both a Windows version and a command-line interface version.
| |
| Lots of atomic orbitals, arrange by quantum number and shape. This table should make the orbital structure more obvious. I believe that it is the most complete orbital table anywhere. |
A Brief Overview
The electron orbitals presented here represent a volume of space within which an electron would have a certain probability of being based on particular energy states and atoms. For example, in a simple lowest-energy state hydrogen atom, the electrons are most likely to be found within a sphere around the nucleus of an atom. In a higher energy state, the shapes become lobes and rings, due to the interaction of the quantum effects between the different atomic particles. In addition to technical merits, they make pretty pictures.The shape of the orbital depends on many factors. The most important are the quantum numbers associated with the particular energy state. These are n, the principal quantum number, l, the orbital quantum number, and m, the angular momentum quantum number. The following table shows some of these shapes. Also available is the Grand Table, showing many, many more orbitals in six different organizations.
| n=1,l=0 | n=2,l=0 | n=2,l=1 | n=3,l=0 | n=3,l=1 | n=3,l=2 | n=4,l=0 | n=4,l=1 | n=4,l=2 | n=4,l=3 | |
| m=0 |  |
 |  |
 |  |  |
 |  |  |  |
| m=1 |  |
 |  |
 |  |  |
||||
| m=2 |  |
 |  |
|||||||
| m=3 | 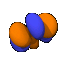 |
 These shapes continue on infinitely, getting ever more lobes or rings on them. Although the l=0, m=0 orbitals look like simple spheres, regardless of n value, this is not actually the case. To the right is a cutaway of a 4s0 (n=4, l=0, m=0) oribital, showing that it is really concentric spheres.
These shapes continue on infinitely, getting ever more lobes or rings on them. Although the l=0, m=0 orbitals look like simple spheres, regardless of n value, this is not actually the case. To the right is a cutaway of a 4s0 (n=4, l=0, m=0) oribital, showing that it is really concentric spheres.A note about the drawings: All of the pictures on this page were produced by the program Orbital Viewer, written by myself. The blue color indicates a positive phase, while the orange color indicates a negative phase, with the phase taken as defined by Condon and Shortley. The colors become important when molecular orbitals are computed.
 So far, all of the pictures have been of electron orbitals associated with a single atom. Molecules can become much more complicated. When two atoms are within a certain proximity of each other, the orbital probabilities can either reinforce each other or cancel each other out. If the phase is the same sign (the same color), the probabilities are reinforced. To the right is a picture of the bonding orbit for H2O (water).
So far, all of the pictures have been of electron orbitals associated with a single atom. Molecules can become much more complicated. When two atoms are within a certain proximity of each other, the orbital probabilities can either reinforce each other or cancel each other out. If the phase is the same sign (the same color), the probabilities are reinforced. To the right is a picture of the bonding orbit for H2O (water).If you wish to see more atomic orbitals, here are four MPEG video files of orbitals rotating. They are 5D0.MPG, 4F0.MPG, 5F0.MPG, and 5G4.MPG. These range from 500 to 750 kb.
PLASMA:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||















No comments:
Post a Comment